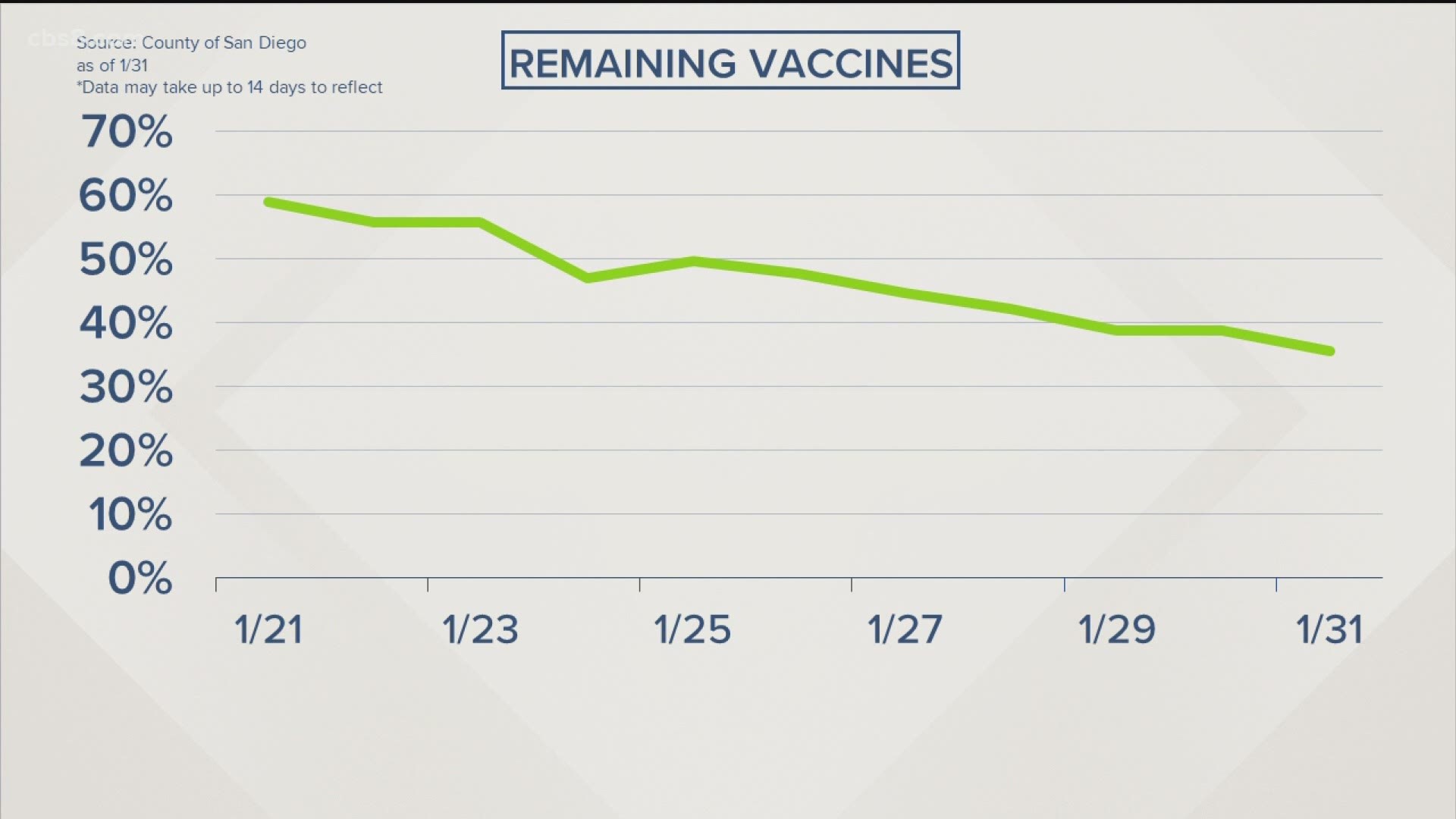
SAN DIEGO COUNTY, Calif. — San Diego County residents do not get to select which vaccine they will receive when visiting a county-funded vaccine site. Officials said supply remains limited and irregular shipments would make it difficult to allow preferences. Once doses arrive, they're quickly listed in the appointment system.
“You don't know what's coming and you have a lot of logistics around ensuring first and second dose or the same vaccine. If folks really are like, ‘well, I only want this one,’ they can go to their health care provider,” explained Supervisor Nathan Fletcher.
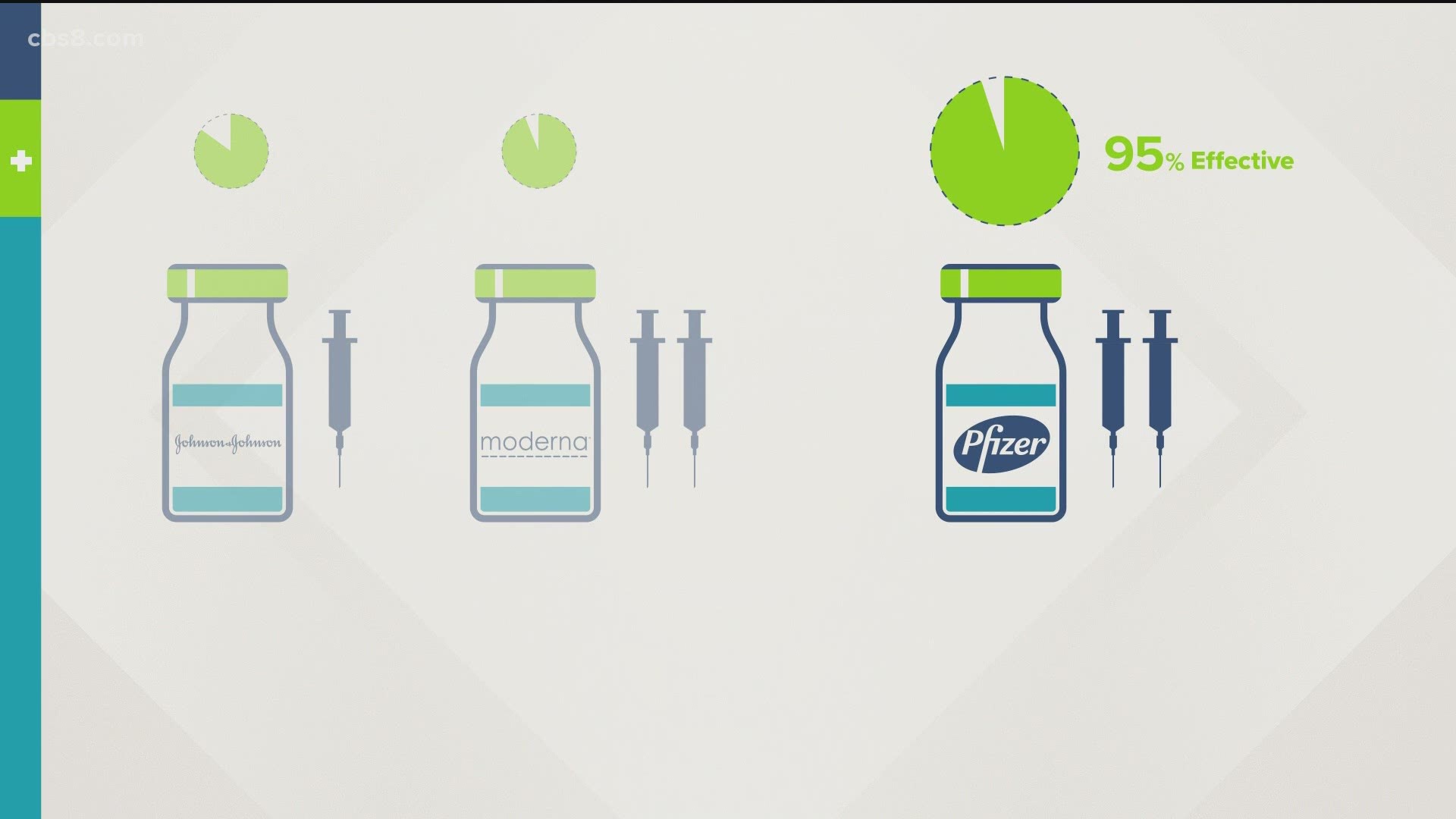
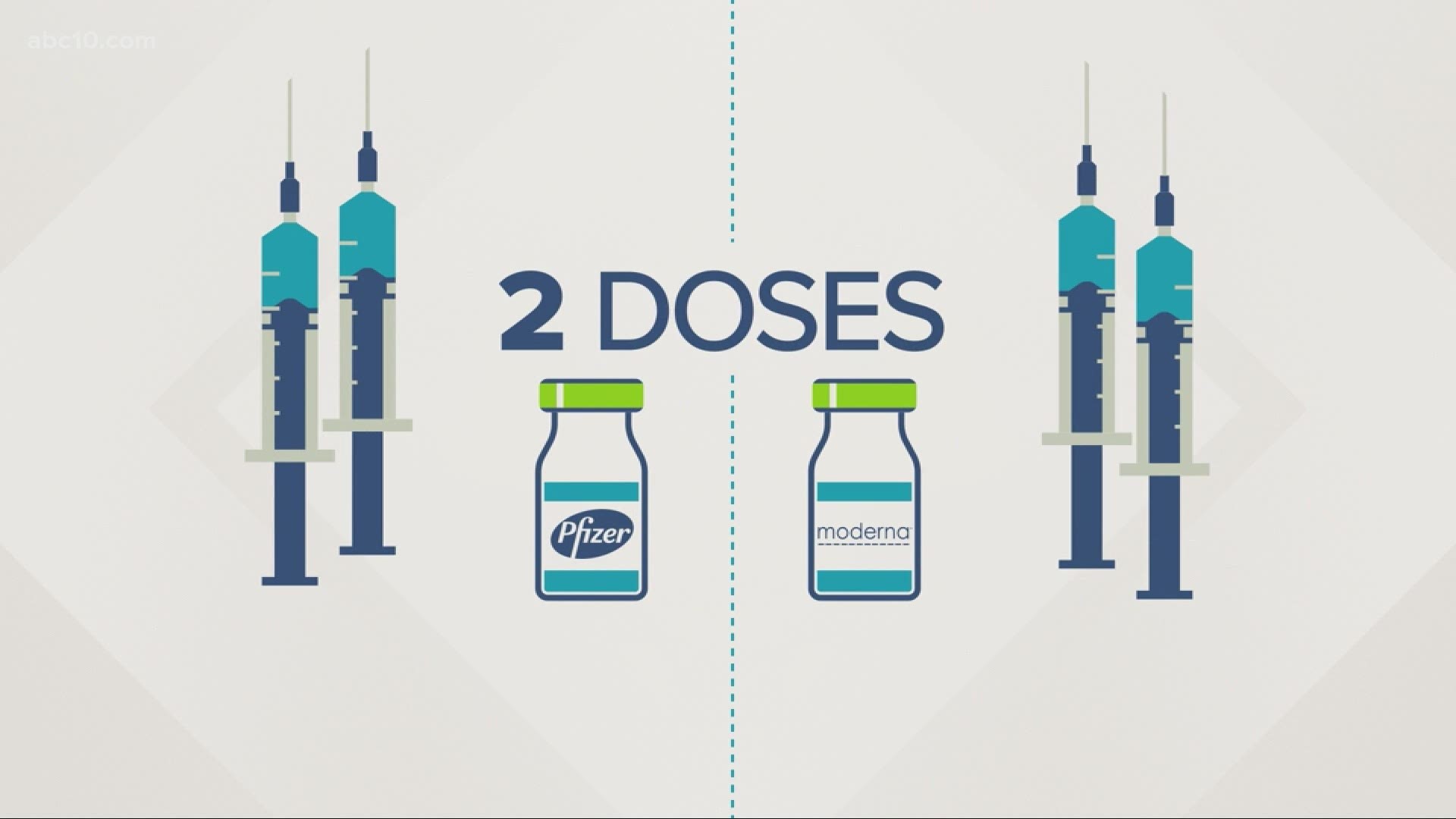
There are only two vaccines approved for use against COVID-19. Both Pfizer and Moderna require two shots either 21 or 28 days apart, respectively. Pfizer was 95% effective during trials while Moderna was 94% effective.
Usually, when making an appointment, users are told which dose they can expect to receive, which could allow those who have a preference to select a site based on availability.
As of Monday afternoon, appointment data showed Pfizer’s vaccine was being offered at Sharp Grossmont this week. While Moderna was available at the South Bay, Petco Park and Coronado Hospital sites. Data for other sites was not available. However, officials stressed future doses at these sites could change based on future shipments although Petco Park has almost always offered Moderna’s since it opened last month.
Moderna requires normal freezer storage and lasts longer once thawed while Pfizer’s needs ultra-cold storage and only lasts a few hours once thawed, which can present a logistical challenge for locations outside of hospitals.
Nationwide, officials are waiting for Johnson & Johnson to submit its vaccine for approval from the U.S. Food and Drug Administration. Data from its Phase 3 trial, which included about 2,000 participants in San Diego County, was released Friday. It showed the vaccine is 72% effective in the U.S. and 66% effective worldwide at preventing moderate to severe COVID-19 symptoms. It was 85% effective in preventing severe illness.
Unlike the previous two vaccines, Johnson & Johnson requires only one shot and normal refrigerator temperatures, which makes it substantially easier to distribute.
Analysts expect the pharmaceutical to submit its paperwork in the next few weeks to the FDA. It took the agency about three weeks to approve both Pfizer and Moderna’s vaccine.