SAN DIEGO — In 2022, the FDA said they wanted to do more to attract diverse participants to clinical trials. The goal came after the White House said there was a disproportionate disease burden for under-represented groups. That means, people of color are being hit harder by diseases simply because medicines and treatments haven't effectively been tested on black and brown populations.
"I had to make a decision for myself and for and for my children, who were at the time, 9, 4 and 3. As a single mom, I had to choose life," Tammy Barnes sayod.
Barnes said 25 years ago she took a chance on a clinical trial after a HIPPA violation in her small town made her health public knowledge. Now, Tammy says she still participates in trials because she's proof, they can work. And she says, she gets a more hands on approach with her doctors.
“If something didn't work, they were on it. If I needed to be hospitalized, I was immediately hospitalized and monitored around the clock. It was nice to sit at the table with the doctors in charge of my care. My one doctor, he's the chief investigator and if I text him, I get a response," Barnes said.
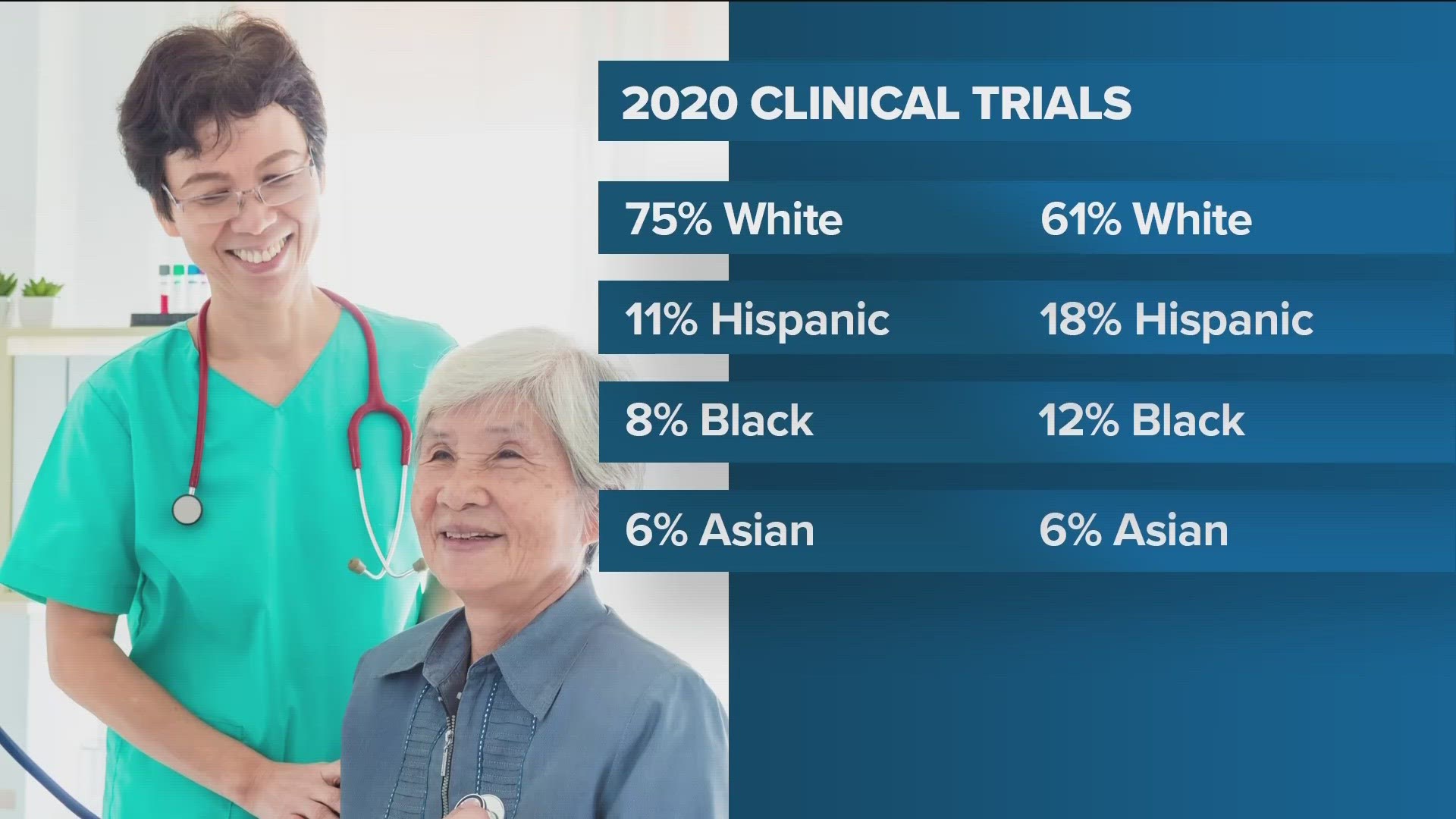
The FDA says in 2020, 75% of clinical trial participants were white. 11% were Hispanic, 8% were Black and 6% were Asian.
But the US Census shows the overall population is 61% white,18% Hispanic, 12% Black and 6% Asian.
Dr. Eddilisa Martin says that's a problem. "The people that participate in clinical trials are not truly representative of the people who will be taking the drug," Martin said.
That's why she's the CEO of M&B Sciences. It's her job to increase diversity in medical clinical trials.
Martin says it's an uphill battle. "Not just in clinical trials but in healthcare, there have been disparities and injustice that really has been perpetuated for hundreds of years. Everybody reflects back to the Tuskegee Experiment. So many things have been put in place to protect people. All of the protections that have been put in place for people participating in clinical trials, the informed consent, the regulatory bodies, things have really changed," she said.
Late last year, public law 117-328 was passed. It requires diversity action plans for clinical trials used by the FDA to decide whether drugs are safe and effective.
Martin says that's a great start for her to come and bridge the gap for communities of color and cutting-edge healthcare. She says clinical trials could really help under insured or uninsured patients.
"Clinical trials can also be an option for care. Diabetes or high blood pressure or any type of cancer, you're not going to get a sugar pill or placebo. You'll get at least the standard of care," Martin said.
Barnes says, she prefers the level of care she's getting in clinical trials. When it comes to getting more diverse representation, She shared, "It's vital to the study of care. The whole person is being studied in a clinical trial for the benefit of the next person."
It’s not just racial diversity, trials need age and location diversity – whether participants live in urban or rural spaces – as well as a myriad of other factors.
This is a big decision for any patient or family to consider. If you'd like to get more information about diverse clinical trials, click here.
If you'd like to see if there's a clinical trial in your area, click here.
For more information on M&B Sciences, click here.
WATCH RELATED: Chidibere Ibe changing the world of medicine one illustration at a time